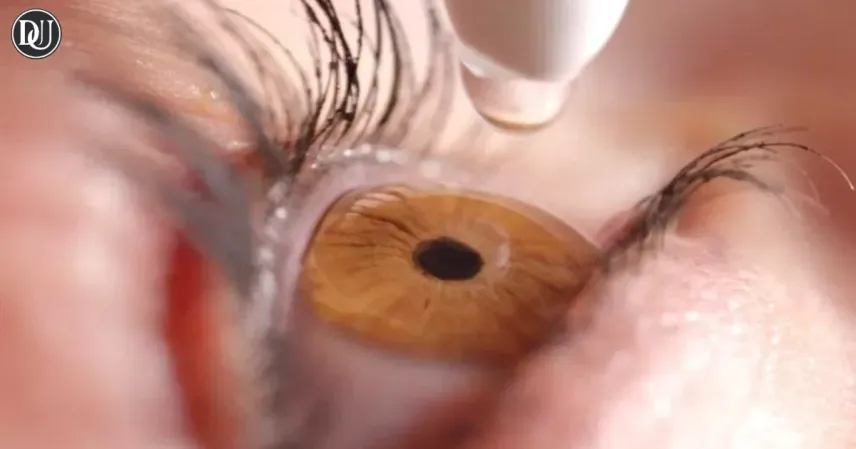
A nationwide eye care product recall has been issued for several over-the-counter eye care products distributed by AvKARE due to concerns over sterility and manufacturing issues. The recall, announced by the U.S. Food and Drug Administration (FDA), affects approximately 1.8 million cartons of eye care products, including Artificial Tears Ophthalmic Solution, Carboxymethylcellulose Sodium Ophthalmic Gel 1%, Carboxymethylcellulose Sodium Ophthalmic Solution, Lubricant Eye Drops Solution, and Polyvinyl Alcohol Ophthalmic Solution.
The eye care product recall was triggered after an FDA audit revealed unspecified manufacturing deviations that could compromise the sterility and safety of the products. While no specific health risks have been confirmed, the FDA has emphasized that the use of the affected products could lead to eye infections or other health complications. The recall includes products distributed nationwide between May 26, 2023, and April 21, 2025.
Consumers who have purchased the affected eye care product recall items are urged to stop using them immediately. AvKARE has stated that they are working closely with the FDA to resolve the issue and ensure that the products meet safety and sterility standards. As part of the eye care product recall process, AvKARE is offering full refunds, including shipping costs, to consumers who return the affected products. Customers are encouraged to complete the return forms provided by the company to facilitate the return and refund process.
The eye care product recall has drawn attention to the importance of maintaining rigorous manufacturing practices for products that come into direct contact with sensitive areas of the body, such as the eyes. Consumers are advised to follow the eye care product recall instructions carefully and consult a healthcare provider if they experience symptoms such as eye irritation, redness, or discomfort after using the affected products.
AvKARE has assured customers that they are working diligently to address the concerns raised by the eye care product recall and to prevent similar issues from arising in the future. The eye care product recall serves as a reminder of the critical need for quality assurance in the production of health-related products.
Consumers who wish to return their recalled products can visit the official AvKARE website for detailed instructions on how to proceed. The eye care product recall highlights the importance of staying informed about potential risks associated with over-the-counter health products and taking immediate action when necessary.
For those who have used the affected eye care product recall items, it is advisable to contact a healthcare provider to assess any potential impact. As the situation develops, further updates on the eye care product recall will be provided by AvKARE and the FDA. To stay informed, consumers are encouraged to monitor the official AvKARE recall page and follow the company’s instructions carefully. The eye care product recall underscores the importance of safety and vigilance when using over-the-counter health products.